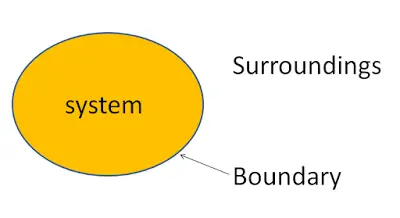
What is Thermodynamic System?
A thermodynamic system is defined as a quantity of matter or a region in space which is selected for the study. The mass or region outside the system is called surroundings. The real or imaginary surfaces which separates the system and surroundings is called boundary. The boundary of a system can be fixed or movable.
To understand the system, surroundings and the boundary in far better way let’s take an example. Considered a closed vessel on which we are going to do our study. Since we are doing our study on the vessel so it is the system and the region excluding the closed vessel is called as surroundings. The surface of the closed vessel that separates the vessel and surroundings is called boundary. Finally anything on which we pay our attention for the study or analysis is called system.
Types of Thermodynamic System
On the basis of mass and energy transfer the thermodynamic system is divided into three types.
1.Closed system
2.Open system
3.Isolated system
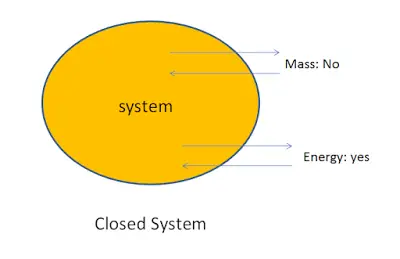
1. Closed system:
A system in which the transfer of energy but not mass can takes place across the boundary is called a closed system. The mass inside the closed system remains constant.
For example: Boiling of water in a closed vessel. Since the water is boiled in a closed vessel so the mass of water cannot escape out of the boundary of the system but heat energy continuously entering and leaving the boundary of the vessel. It is an example of a closed system.
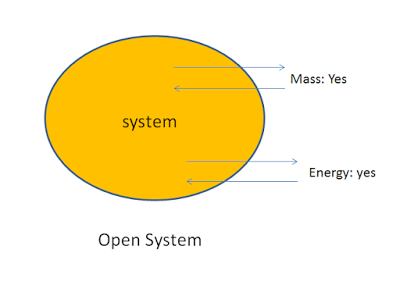
2. Open System:
A system in which the transfer of both mass and energy takes place is called an open system. This system is also known as control volume.
For example: Boiling of water in an open vessel is an example of open system because the water and heat energy both enters and leaves the boundary of the vessel.
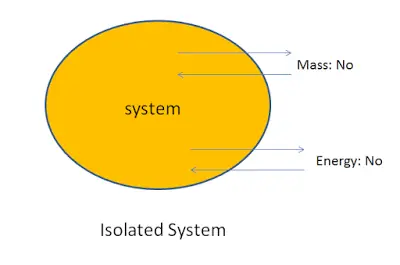
3. Isolated system:
A system in which the transfer of mass and energy cannot take place is called an isolated system.
For example: Tea kept in a thermos flask. In this, the heat and the mass of the tea cannot cross the boundary of the thermos flask. Hence the thermos flak is an isolated system.
Here we have studied about what is thermodynamic system and different types of thermodynamic system. whatever we have discussed above keeps an important role in the study of subject thermodynamic. If anyone does not know about these topics then it becomes very difficult to understand the thermodynamic subject.
If you found this piece of information valuable and useful then don’t forget to like and share it.





.bmp)



