In this article, we will learn about the difference between intensive and extensive properties. Any property may be either intensive or extensive. Before discussing these properties let’s come to know about what is property. Any characteristic of a system is called property. For example: pressure, temperature, volume and mass are some familiar properties of a system. Viscosity, thermal conductivity, modulus of elasticity thermal expansion coefficient, electrical resistivity etc are less familiar properties.

Properties are considered to be either intensive or extensive. Let’s discuss about intensive and extensive properties one by one
Intensive Properties:
The properties which are independent of the mass of the system. Temperature, pressure and density are the intensive properties.
Extensive Properties:
The properties which depend on the size or extent of the system are called extensive properties. For example: total mass, total volume and total momentum.
Also Read:
- Properties of Fluids in Fluid Mechanics
- Thermodynamic System – Types of Thermodynamic System
- Difference Between Thermosetting and Thermoplastic:
For better explanation of the intensive and extensive properties considered a system in which the properties like mass, volume temperature, pressure and density are denoted by ‘m’, ‘V’, ‘T’, ‘P’, ‘ρ’.If the system is divided into two parts then what changes takes place is shown in the diagram below.
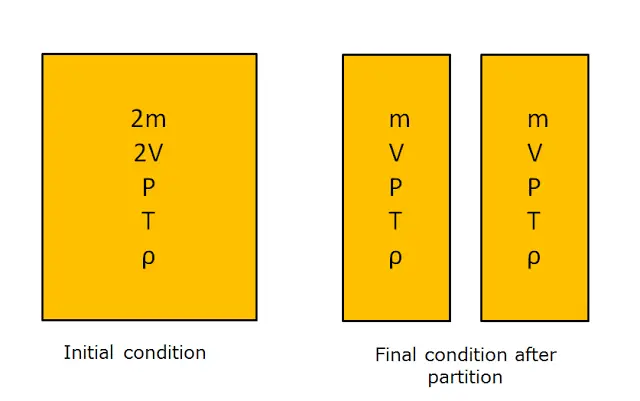
Here the mass and volume becomes half but the pressure temperature and density remains unchanged as the system is divided into two halves. This indicates that mass and volume depends upon the size or extent of the system and hence they are extensive properties. And the pressure, temperature and density remains unchanged that is they are independent of the mass and that’s why are called intensive property.
Difference Between Intensive and Extensive Properties
|
S.no |
Intensive property |
Extensive property |
| 1. | They are independent of the quantity of the system. | They depend upon the quantity of the system |
| 2. | They are independent of the size or extent of the system. | They depend upon the size and extent of the system. |
| 3. | Pressure, temperature and density are intensive properties etc | Total Mass, total volume, total momentum etc. |
How to Identify Whether a Property is Intensive or Extensive
Considered a system and measure some of its properties like mass, volume, temperature density etc.
Note down the value of each property on a paper. Now make the quantity of the system double. Now again measure all the properties of the system that we have measured earlier. And compare the values
- If the value any property exchanges than it is an extensive property
- And if it remains unchanged than it is an intensive property.
This is all about the difference between intensive and extensive properties. If you found this piece of information valuable and useful then don’t forget to like and share it.








Extensive properties are additive where as intensive properties are not additive