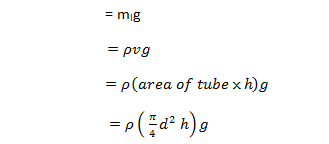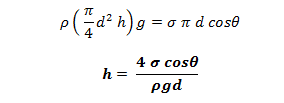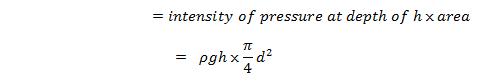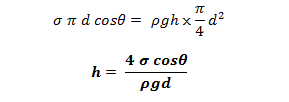- Specific weight of the liquid.
- Diameter of the tube and
- Surface tension of the liquid.
Examples Based on What is Capillarity or Capillary Action
- In plants the rise of water from the roots to all its parts takes place because of capillary action.
- The capillary action draws ink to the tips of a fountain pen from cartridge (reservoir) inside the pen.
- The towels that we use after taking bath, absorb water from our body because of capillary action.
- Sponge which has larger number of small pores acts as small capillaries and absorbs a large amount of water.
- The cotton clothes that we wear in hot summer day shows capillary action and absorbs all our body sweat and maintains the temperature of the body to normal.
Expression for Capillary Rise
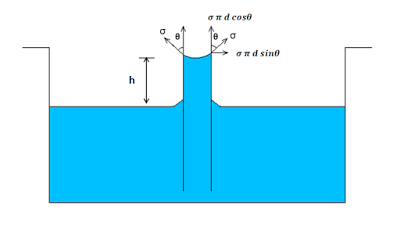
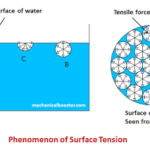
In order to find expression for the capillary rise, let us take a small tube having diameter ‘d’ opened at both ends. It is inserted in a liquid say water. Gradually the rise of the liquid takes above the level of the liquid.
Let h is the height of the liquid that rises in the tube. Under equilibrium condition, the weight of the liquid of height h is balanced by the forces at the surface of the liquid. But the force that acts at the surface of the liquid is due to surface tension of the liquid.
Let
σ = Surface tension of the liquid.
θ = Angle of contact between liquid and glass tube.
The weight of liquid of height h in the tube
From the figure above,
The vertical component of the surface tensile force
Under equilibrium condition, the weight of the liquid in the tube is balanced by the vertical component of the surface tensile force. i.e.
The value of θ in between clean glass tube and water is nearly equal to zero.
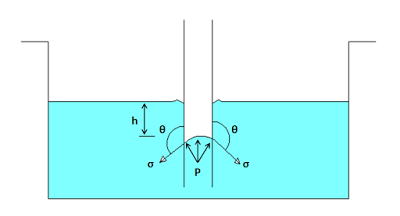
Expression for capillary fall
When the glass tube is dipped in mercury than instead of rising, the level of mercury in the tube goes down than the normal level of the outside liquid. Let the ‘h’ is the height of depression of liquid in the tube.
Under equilibrium condition two forces are acting on the mercury inside the tube. The first force is due to surface tension acting in the downward direction and second force is because of hydrostatic force acting in upward direction.
The force due to surface tension acting downward direction is given by
And the force due to hydrostatic force acting downward is given by
Under equilibrium condition the two forces i.e. the force due to surface tension is equal to the hydrostatic force.
The value of θ for the mercury and glass tube is 128 degree.
In this article we have learnt about what is capillarity and capillary action, we have also discussed about the examples showing capillarity and derived the expression for the rise and fall of liquid in a tube.
If you find anything missing or incorrect than comment us. And it this article has given you some valuable information then don’t forget to like and share it on social networks.