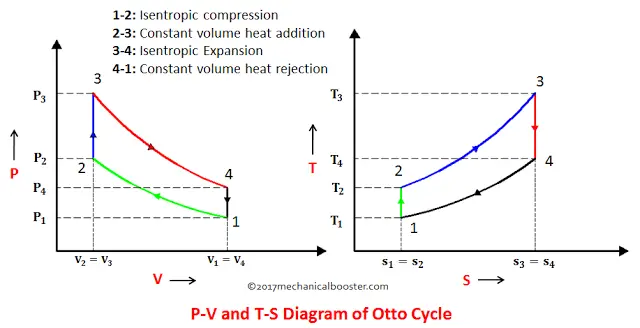
The Otto cycle was given by Dr. Nikolaus August Otto. It is a gas power cycle that is used in spark ignition engine (i.e. petrol engines) for its working. The entire modern petrol engine works on Otto cycle. It consists of four processes, Two isentropic (reversible adiabatic) processes and two isochoric (constant volume) processes. It has a low compression ratio ranging from 7:1 to 10:1. Here we will try to understand this cycle with the help of its P-V and T-S diagram.
The four processes of this cycle are as follows:
1. Isentropic ( reversible adiabatic) compression
2. Constant volume (Isochoric) heat addition
3. Isentropic (reversible adiabatic) Expansion
4. Constant volume heat rejection.
Before starting to understand these four processes, lets us first understand about isentropic and isochoric process.
- Isentropic Process: It is a thermodynamic process in which the entropy of the system remains unchanged (i.e. entropy remains constant). There is no dissipation of heat takes place during isentropic process, so sometime isentropic process called as reversible adiabatic process.
- Isochoric Process: The process that takes place at constant volume is called the isochoric process.
How does Otto Cycle Work?
Now, with the help of the P-V and T-S diagram, we can easily understand all the processes of the Otto cycle.

Also Read:
- Difference Between Otto Cycle and Diesel Cycle
- Difference Between 2-Stroke and 4-Stroke Engines
- Difference Between Tube and Tubeless Tyres
Note: For a better explanation, while reading watch the P-V and T-S diagrams every time.
1. Process 1-2: Isentropic Compression
This process involves the motion of the piston from TDC to BDC. The air that is sucked into the cylinder during suction stroke undergoes reversible adiabatic (isentropic) compression. Since the air is compressed, the pressure increases from P1 to P2, the volume decreases from V1 to V2, the temperature rises from T1 to T2, and entropy remains constant.
2. Process 2-3: Constant Volume Heat Addition
This process is an isochoric process i.e. the heat is added to the air at constant volume. The piston in this process rest for a moment at TDC and during this time heat is added to the air through an external source. Due to the heat addition, the pressure increases from P2 to P3, volume remains constant(i.e. V2=V3), temperature increases from T2 to T3 and entropy increases from S2 to S3.
The amount of heat added is given by

3. Process 3-4: Isentropic Expansion
In this process, the isentropic (reversible adiabatic) expansion of air takes place. The piston moves from TDC to BDC. Power is obtained in this process which is used to do some work. Since this process involves expansion of air, so the pressure decreases from P3 to P4, volume increases from V3 to V4, temperature falls from T3 to T4 and entropy remains unchanged (i.e. S3=S4).
4. Constant Volume Heat Rejection
In this process, the piston rests for a moment at BDC and rejection of heat takes place at constant volume. The pressure decreases from P4 to P1, Volume remains constant (i.e. V4=V1), temperature falls from T4 to T1.
The amount of heat rejected in this process is given by

When this cycle is used in four four-stroke petrol engine than the two process increases. one is the suction of air-fuel mixture inside the cylinder which takes place at constant atmospheric pressure and the other one is the exhaust of gases out of the engine cylinder at constant atmospheric pressure. These two processes are not shown in the ideal Otto cycle that we have discussed above.
Also Read:
- What is Four Stroke Engine?
- What is a Two Stroke Engine and How it Works?
- Diesel Cycle – Process with P-V and T-S Diagram
Summary in Tabular Form
| S.no | Process | Operation | Position of piston | Change in parameter |
| 1. | 1-2: Isentropic Compression | Compression of air. | BDC to TDC | V: Decreases from V1 To V2 T: Increases from T1 to T2 P: Increases from P1 to P2 S: Entropy remains constant (S1=S2) |
| 2. | 2-3: Constant Volume Heat Addition | Heat is added in the form of spark and combustion occurs. | At TDC for a moment | V: Remains constant (V2 = V3 ) T: Increases from T2 to T3 P: Increases from P2 to P3 S: Increases from S2 to S3 |
| 3. | 3-4: Isentropic Expansion | Expansion of air takes place due to heat addition. | TDC to BDC | V: Increases from V3 to V4 T: Decreases from T3 to T4 P: Decreases from P3 to P4 S: Entropy remains constant (S3=S4) |
| 4. | 4-1: Constant Volume Heat Rejection | Heat is rejected to a sink. | At BDC for a moment | V: Remains constant(V4 = V1 ) T: Decreases from T4 to T1 P: Decreases from P4 to P1 S: Decreases from S4 to S1 |
Thermal Efficiency
The efficiency of Otto cycle is given by

Application
It is used in all two-stroke and four-stroke petrol engines of motorcycles, cars, and other light-duty vehicles.
Conclusion
In this post, we have discussed what is Otto Cycle with their PV and TS Diagram. I hope you have understood it clearly and If you find this piece of information useful and valuable then don’t forget to like and share it.